advice and support
MITeC research project
MITeC is open to research projects with researchers from any institution or company. We can offer advice, carry out research, give training or open up our operating rooms to researchers.
read moreMITeC research project
MITeC is open to research projects with researchers from any institution or company. We can offer advice, carry out research, give training or open up our operating rooms to researchers.
In the timeline on this webpage you can discover what we can offer at all stages of research into medical devices and/or treatments. We can oversee research from idea to assessment and even long-term surveillance. But it is also possible to join forces in just one or a couple of these stages. We are also open to any suggestions you yourself have for research collaborations.
In short, these are the stages:
- Preclinical (IDEAL stage 0)
- Proof of concept/First in human (IDEAL stage 1)
- Development (IDEAL stage 2)
- Exploration (IDEAL stage 2)
- Assessment (IDEAL stage 3)
- Long-term (IDEAL stage 4)
Contact
Interested in any of the research or training possibilities MITeC has to offer? Please contact us. send an email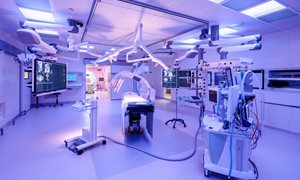
About MITeC
MITeC offers unique consulting services and expertise to incorporate newly developed medical technology innovations and accelerate their success rate.
read moreAbout MITeC
MITeC, which stands for Medical Innovation and Technology expert Center, groups together researchers, surgeons, technicians and other medical experts. At MITeC, the efficacy, efficiency, safety, and feasibility of innovative surgical procedures are assessed in studies. We further develop those procedures that have proven to have added value to patient care.
This MITeC environment is unique in Europe because of the combination of technology development and determination of clinical relevance by means of evidence-based surgery.
Connected operating rooms
The MITeC facilities consist of two operating rooms that are interconnected by an MRI room. In this MRI room, MRI guided procedures can be performed – if needed also under anesthesia. One of the two connected operating rooms is a typical OR, while the other is a hybrid OR including a ZEEGO system, a robotic-assisted positioning capability for interventions designed to offer greater flexibility of movement and image acquisition.
Improving patient care
As the operating rooms are interconnected, patients can easily be transported from one imaging modality to another, or from the operating table to an imaging modality. So, be transported from, for example, the hybrid OR to the MRI. The main advantage of this, is that a patient can get the necessary scans during an operation allowing surgeons to operate more precisely and immediately be able to assess whether more intervention is needed. This increases the success rate as well as diminishing the need of a second operation. It’s less stressful for patients as well as being more cost effective.
Research
Thanks to MITeC, our experts have already discovered ways on improving several surgical procedures. The research groups that use our facilities give a good indications of the research that is conducted at MITeC.
In order to enable new discovers, the MITeC facilities are open to researchers outside of Radboudumc. We are also open to collaborating with other researchers and organizations.
Current research
Want examples of MITeC research? Have a look at what some research groups connected to our technology center are doing. We have also done collaborative research with several companies (e.g. Siemens Healthineers, Profound, Medical and New Compliance).
Facility Innovative operating rooms
Embedded within the Radboudumc central OR suite, three MITeC operating rooms have truly unique layout and equipment. go to pagePreclinical
screening
Preclinical phase IDEAL stage 0
Do you have an inkling of an idea but not sure where to start? Do you want to know if your idea for a medical device or treatment is feasible? Want to know the (financial) risks to increase impact and profit? Then we can help.Possible outcomes of this phase
- Early HTA report
- Early technology report
- Business case
- Systematic review
- Stakeholders analysis
- Simulation studies
- Animal studies
- Anatomic lab
Possible outcomes of this phase
In this phase we can offer you the following options:- Early Health Technology Assessment report which will, among others, determine at an early stage whether your innovation will be (cost) effective and will really result in health gains for patients when compared to current available treatments.
- Early technology report which will, among others, determine at an early stage XXX.
- Business case which will, among others, determine if there is sufficient potential within the market to successfully market the end product.
- Systematic review in which we answer a defined research question by collecting and summarising empirical evidence that fits pre-specified eligibility criteria. Radboudumc has extra expertise in systematic reviews of animal studies.
- Stakeholders analysis which will analyse if your innovation will be accepted by all stakeholders involved (like patients, medical staff, insurers), or what is required for this to happen.
- Simulation studies
- Animal studies
- Anatomic lab
Collaborations
MITeC is a Radboudumc technology center (RTC) with its own area of expertise. In pursuit of research we collaborate with the many other RTCs. In this phase we often collaborate with the following ones…Contact
Interested in any of the research or training possibilities MITeC has to offer? Please contact us. send an emailFirst in human
proof of concept
First in human phase IDEAL stage 1
When you have done all the necessary research in the pre-clinical phase it is time for the proof of concept. read moreFirst in human phase IDEAL stage 1
At Radboudumc we have the expertise and know-how of on how to do this in human patients for the first time. Our team of researchers has plenty of scientific experience of past research projects as well as creativity to come with ideal solutions on any medical question presented to us.This is the phase in which well-founded go/no-go can be given to an innovation.
Possible outcomes of this phase
- Protocol for feasibility study
Contact
Interested in any of the research or training possibilities MITeC has to offer? Please contact us. send an emailDevelopment
technical success / safety
Development phase IDEAL stage 2A
After the preparatory phases, comes the point to put theory to the test. Can a surgeon perform the medical treatment and/or use your medical device? read moreDevelopment phase IDEAL stage 2A
After the preparatory phases, comes the point to put theory to the test. Can a surgeon perform the medical treatment and/or use your medical device?A small feasibility study is preformed with 2 to 5 cases. Technique and procedure are analyzed. What is the rate of success? What about the patient’s safety?
The conclusion of the phase is also another protocol for feasibility study. Now instead of simply asking: is it possible, the question lies with whether surgeons of all levels could be able to perform the necessary procedure.
Possible outcomes of this phase
- Technical report
- Protocol for feasibility study
Contact
Interested in any of the research or training possibilities MITeC has to offer? Please contact us. send an emailExploration
clinical outcomes
Exploration phase IDEAL stage 2
When it is has been determined that a procedure is possible, the question turns to the possibility of implementing a medical treatment or device on a large scale. read morePossible outcomes of this phase
- Feasibility outcomes
- Protocol (ethical committee)
- Mock-up session
Training
Do you have a treatment that has already be deemed viable? Our facilities can be used to train other surgeons.Protocol
We can help set up a protocol that is needed for clinical studies and mandated by the ethical committee.Mock-up sessions
In a so-called ‘mock-up’ session the complete surgical and supportive team rehearses the procedure with a dummy patient in real-life circumstances, looking at aspects such as patient flow, logistics, planning and communication.Contact
Interested in any of the research or training possibilities MITeC has to offer? Please contact us. send an emailAssessment
clinical studies
Assessment phase IDEAL stage 3
Clinical studies are an ideal way to assess a treatment. At Radboudumc we have plenty of expertise when it comes to various types of clinical studies. read moreAssessment phase IDEAL stage 3
Clinical studies are an ideal way to assess a treatment. At Radboudumc we have plenty of expertise when it comes to various types of clinical studies, like comparative effectiveness, randomized controlled trails and adaptive clinical trials. Of course, cost effectiveness can also be assessed.Possible outcomes of this phase
- Project management
- Data-management
- Monitoring
- Reporting
- (Statistical) analyses
Network
Besides the necessary expertise, we also have the network to conduct large scale clinical trials.Training
Do you have a treatment that has already be deemed viable? Our facilities can be used to train other surgeons.Collaborations
MITeC is a Radboudumc technology center (RTC) with its own area of expertise. In pursuit of research we collaborate with the many other RTCs. In this phase we often collaborate with the following ones…Contact
Interested in any of the research or training possibilities MITeC has to offer? Please contact us. send an emailLong-term
surveillance / registries
Long-term phase IDEAL stage 4
In many countries registration of medical data has become mandatory. Therefore, at Radboudumc we have begun to specialize in not just the criteria but also IT possibilities of such registrations. read moreLong-term phase IDEAL stage 4
Finally there is the long-term phase. In many countries registration of medical data has become mandatory. Therefore, at Radboudumc we have begun to specialize in not just the criteria but also IT possibilities of such registrations.We can help you set up a repository and data registration. We can also offer to house your data. One of the innovations we have created to be able to do this is DRE, the Digital Research Environment. Finally there is the long-term phase. In many countries registration of medical data has become mandatory. Therefore, at Radboudumc we have begun to specialize in not just the criteria but also IT possibilities of such registrations.
We can help you set up a repository and data registration. We can also offer to house your data. One of the innovations we have created to be able to do this is DRE, the Digital Research Environment.
Possible outcomes of this phase
- Registry
- Routine database

What is the Digital Research Environment?
The Digital Research Environment (DRE) is a cloud based, globally available research environment where data is stored and organized securely and researchers can quickly generate workspaces to collaborate in and use the applications they love. Globally available and accessible 24/7. read moreWhat is the Digital Research Environment?
The Digital Research Environment (DRE) is a cloud based, globally available research environment where data is stored and organized securely and where researchers can quickly generate workspaces to collaborate in. Within these workspaces, researchers have preinstalled applications at their disposal, as well as the ability to bring own tooling. Globally available and accesible 24/7.The DRE facilitates users to collaborate on research projects in a safe, yet flexible compute and storage environment. The architecture of the DRE allows researchers to use a solution within the boundaries of data management rules and regulations. Although General Data Protection Regulation (GDPR) and Good (Clinical) Research Practice still rely on researchers, the DRE offers tools to more easily control and monitor which activities take place within your projects.
Full control
Within the DRE platform each of the projects you are a member of consists of a separate, secure folder, called a 'workspace'. Each workspace is completely secure, so as a researcher you are in full control of your data. You can invite (external) colleagues into your workspace for collaboration, and with the same ease revoke access at any time. Each workspace has its own list of users, which can be managed by its administrators.Scalable
Each workspace is fully scalable with regard to data quantity and computing power, thereby supporting anything from small to complex multicenter, multisource studies. Moreover, the workspace enables you to perform ‘worry free’ research, meaning that security, ICT infrastructure and compliance with laws and regulations are automatically taken care of when using the DRE.Various sources
You can handle many data types from various sources within your workspace. Some examples: data collected with a survey tool or an electronic data capture tool like Castor or RedCap, data from measuring equipment, IKNL data, clinical data from Biobanks and omics data.Install your own tooling
The DRE consists of a web-interface enabling access to virtual machines. Preinstalled tools consist of programs such as SPSS, R Studio, Matlab, and Office, but you are free to install your own tools. Because of the security boundary around workspaces, it is possible to offer users the ability to install their own tooling without harming others.Thanks to the flexibility of the Azure cloud platform, on which the DRE has been built, we can offer a variety of virtualized hardware, including, but not limited, to:
- Standalone virtual Windows / Linux machines (e.g. 72 cores, 144 Gb RAM);
- Compute Clusters (HPC);
- Web-Servers;
- Several storage options (tables/files/SQL/etc.).
DRE consists of a web portal, where, for each workspace separately, you can up- and download data, do member management and connect to virtual machines (launched by a simple click on the icon on your own desktop, like a computer in a computer).
